An updated COVID booster to target omicron? What to know as subvariants spread
Will the makeup of COVID-19 booster vaccines be modified to target the rapidly evolving omicron variant?
It’s possible as Food and Drug Administration advisers — the Vaccines and Related Biological Products Advisory Committee — recommended adjusting the shots on Tuesday, June 28, for the upcoming fall and winter seasons.
The expert panel discussed how protection offered by the original vaccines wanes with time and is “less robust” when it comes to protecting against coronavirus variants currently spreading. Because of this, an adjustment is likely needed.
Here’s what to know as omicron’s subvariants dominate all COVID-19 cases in the U.S., according to Centers for Disease Control and Prevention data estimates.
“Since omicron came on the scene … we have seen the BA.1 variant, which was initially what omicron was circulating as,” is “no longer circulating. … It has been eclipsed by other omicron variants,” Dr. Peter Marks, a top FDA official in charge of vaccine regulation, noted during the panel’s discussion.
Omicron’s latest subvariants on track to become most dominant
CDC data estimates show that the BA.2.12.1 omicron subvariant makes up nearly half of COVID-19 cases in the U.S. as of June 25.
Meanwhile, two newer subvariants — BA.4 and BA.5 — make up more than half of cases and have spread more widely over the past several weeks, according to the CDC.
BA.4 and BA.5 were characterized as “escape artists,” which are “highly evasive against past immunity” by Dr. Eric Feigl-Ding, an epidemiologist, health economist and head of the COVID Risk Task Force at the New England Complex Systems Institute, on June 29.
NEW DOMINANCE of “ESCAPE ARTIST” Variants #BA5 & #BA4–together topping 52% of all strains in the US, all while #BA2 family going extinct. ️BA5&4 are highly evasive against past immunity—matches rise in #COVID19 hospitalization in US & many countries. https://t.co/amAjDWufhr pic.twitter.com/s263LSfDo4
— Eric Feigl-Ding (@DrEricDing) June 29, 2022
During the FDA committee meeting, Marks expressed concern over how these subvariants could soon become the most dominant virus strains throughout the country and how the possibility of a COVID-19 surge in the winter is likely as Americans stay in indoor spaces more often.
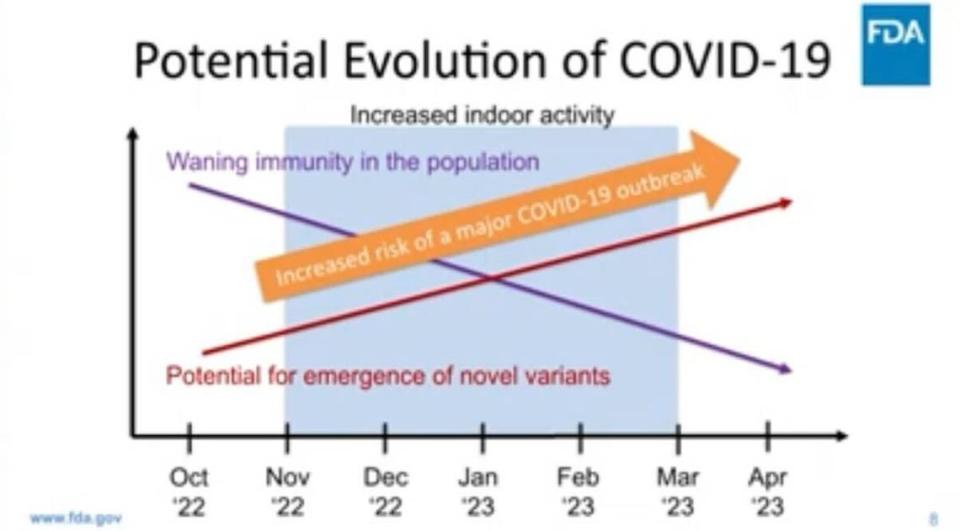
The concern also comes from how roughly half of Americans who received their first booster dose (third dose) likely have “waning immunity” by now, Marks said.
After the panel’s discussion, the FDA committee members voted 19-2 to recommend adjusting booster shots for the fall so they can combat variants circulating.
Officials did not discuss how the vaccines would be altered, but experts are weighing whether to change the existing vaccines to target BA.4 and BA.5.
Single shot composition ‘desirable’
The discussion comes after a prior committee meeting took place during which members agreed that a “single vaccine composition used by all manufacturers,” such as Pfizer and Moderna, “was desirable,” McClatchy News previously reported.
Three FDA officials, including Marks, wrote in a paper published in the peer-reviewed journal JAMA on May 2 that the current vaccines are “reasonably good at protecting severe outcomes” when it comes to sickness, but adjusting them may achieve better protection against variants.
COVID-19 “will likely circulate globally for the foreseeable future, taking its place alongside other common respiratory viruses such as influenza,” the officials wrote.
By early July, a recommendation on an updated booster vaccine composition is expected, Marks said.
Yearly COVID shot may be needed as virus becomes ‘new normal,’ FDA officials say
COVID-sniffing dogs can also smell long-term virus symptoms in patients, study says
Keep getting COVID? Each time increases the risk of health complications, study finds
COVID may harm babies’ brain development if mom is infected while pregnant, study says

 Yahoo Movies
Yahoo Movies 
