A recalled emergency blood sugar kit failed. This caused seizures and can cause death
Eli Lilly recalled an emergency kit that can save the lives of people with diabetes having severe hypoglycemia. The reason: “loss of potency” after the kit failed a customer who needed it.
Here’s what you need to know.
What’s recalled?
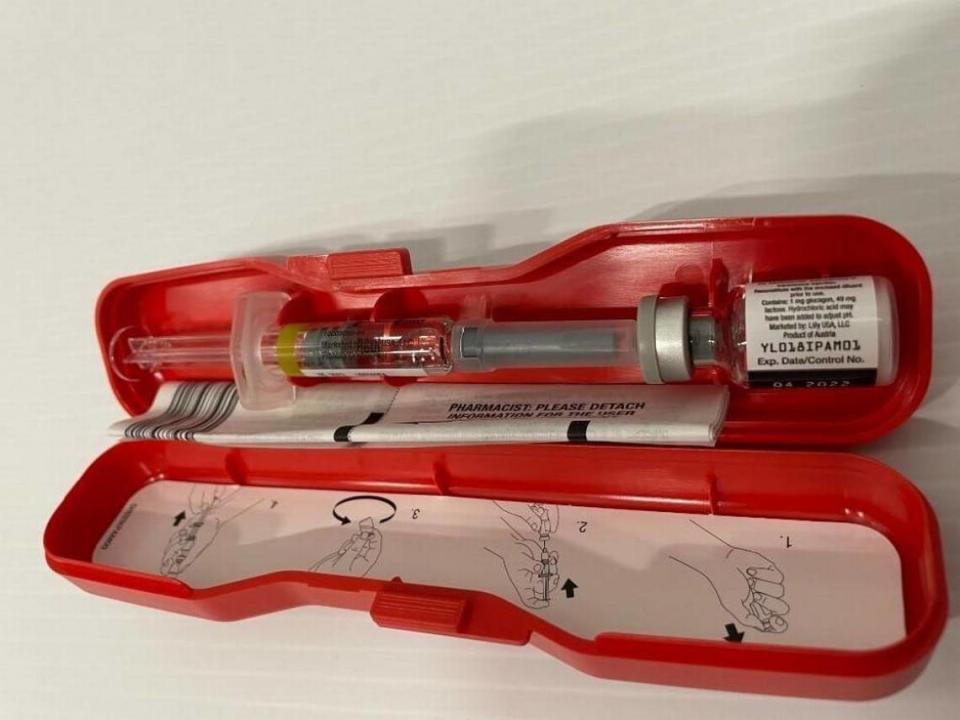
Glucagon Emergency Kit for Low Blood Sugar, lot No. D239382D, expiration date April 2022, expressed on the label as “04 2022.” The lot number and expiration date can be found on the back of the packaging and on a vial inside.


The lot went nationwide.
What’s the problem with this Glucagon kit?
The Lilly-written, FDA-posted recall notice says the Indianapolis company got a complaint that “the vial of Glucagon was in liquid form instead of the powder form.
“The use of the liquid form of this product may fail to treat severe low blood sugar due to loss of potency.”
And the consequences of that failure can go from “transient, minor complaint to neurological damage, seizures and even death if not promptly treated.”
The recall notice says the patient in the complaint had seizures after the drug didn’t work.
What do you do now?
If you’re a wholesaler or pharmacist, call your customers and ask them to contact Sedgwick by phone call at 877-907-7032, fax at 877-884-9410 or by email at elililly7484@sedgwick.com, Monday through Friday, 8 a.m. to 5 p.m., Eastern time for a reply card to return the rest of your lot.
If you’re a patient, call Lilly at 800-545-5979 (LILLYRX), Monday through Friday, 9 a.m. to 7 p.m., Eastern time.
If this or any drug causes a problem, after notifying a medical professional, let the FDA know via its MedWatch Adverse Event page or by filling out a form you can get by calling 800-332-1088. Only then do you notify the drug company.
A recalled antibiotic might not be sterile. That can cause ‘life-threatening’ infections
Pfizer expands recall to all lots of anti-smoking drug Chantix for carcinogen content

 Yahoo Movies
Yahoo Movies 