A recalled antibiotic might not be sterile. That can cause ‘life-threatening’ infections
Eleven lots of the compounded intravenous antibiotic Cefazolin have been recalled after manufacturer IntegraDose Compounding Services said there was “a lack of sterility assurance resulting from compounding in a newly installed biologic safety cabinet without completing dynamic smoke study testing.”
That’s from the IntegraDose-written, FDA-posted recall alert, which posted Tuesday night. Compounded drugs combine two or more drugs and aren’t FDA regulated.
“Intravenous administration of a non-sterile drug could result in serious infections ranging from fever, chills, and malaise, to severe adverse events such as septicemia, bacterial meningitidis and wound infection which may be life-threatening,” the alert said.
“The possibility of a breach in sterility assurance in distributed product, while not confirmed, cannot be eliminated.”
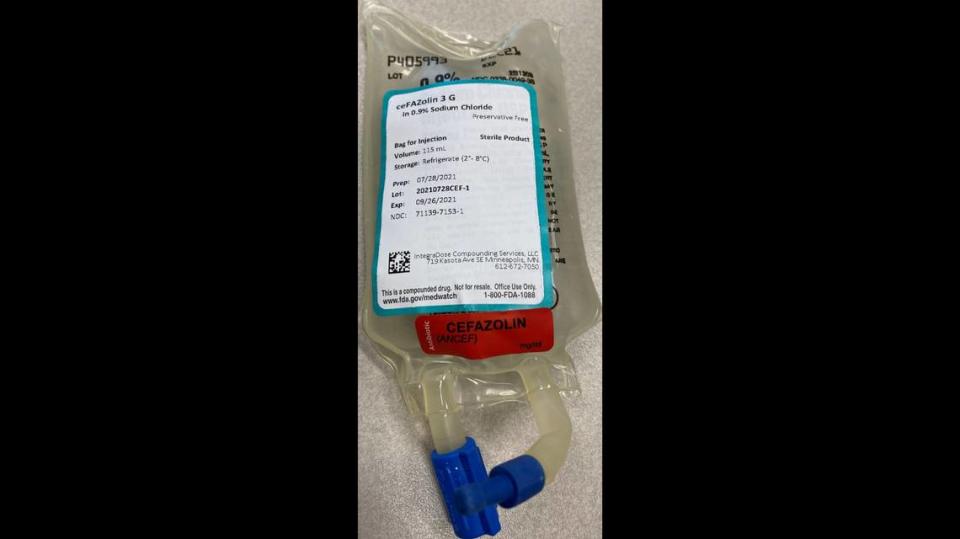
What Cefazolin has been recalled?
▪ Cefazolin 2 gram in 20 mL syringe for injection: lot Nos. 20210803CEF-1, expiration 9/17/2021; 20210805CEF-3, expiration 9/19/2021; 20210806CEF-1 and 20210806CEF-2, expiration 9/20/2021; 20210809CEF-1 and 20210809CEF-2, expiration 9/23/2021; 20210810CEF-1, expiration 9/24/2021; 20210811CEF-1, expiration 9/25/2021; and 20210812CEF-1, expiration 9/26/2021.
▪ Cefazolin 3 gram in 100 mL 0.9% sodium chloride bag for injection: 20210722CEF-2, expiration 9/20/2021; and 20210728CEF-1, expiration 9/26/2021.
They went to hospitals nationwide from Aug. 12 through Sept. 15.
Pfizer expands recall to all lots of anti-smoking drug Chantix for carcinogen content
Medication for kids recalled for mold, yeast and bacterial contamination

What you should do if you have the recalled Cefazolin?
Hospitals or distributors with the recalled drugs should quarantine them now and contact IntegraDose about returning the Cefazolin.
Anyone with questions or a need to contact IntegraDose can email celse1@fairview.org or call 612-672-5216, Monday through Friday, 9 a.m. to 5 p.m., Eastern time.
If this or any drug causes a problem, after notifying a medical professional, let the FDA know via its MedWatch Adverse Event page or by filling out a form you can get by calling 800-332-1088. Only then do you notify the drug company.

 Yahoo Movies
Yahoo Movies 
