Pfizer and BioNTech say lower dose of COVID-19 vaccine proved safe, effective in kids 5 to 11
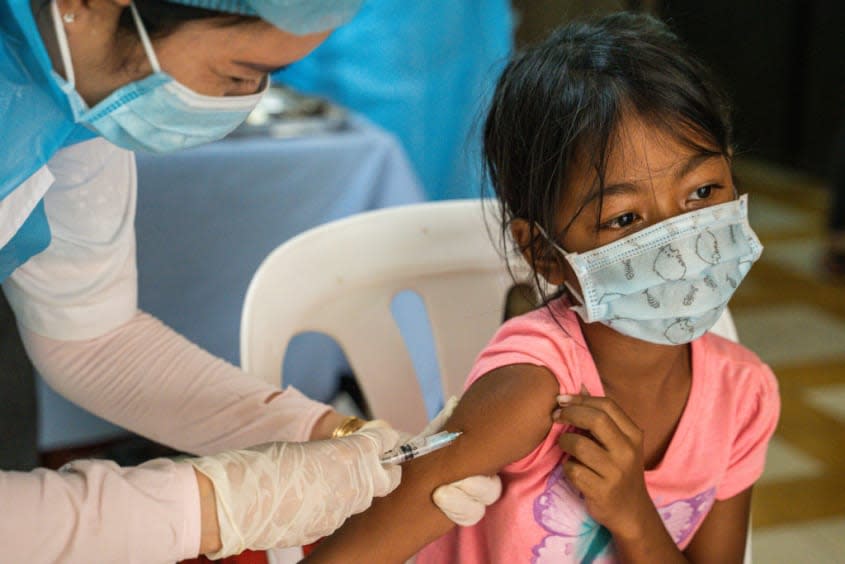
Pfizer and BioNTech said Monday morning that its COVID-19 vaccine was shown to be "safe, well-tolerated, and showed robust neutralizing antibody responses" in children 5 to 11. The 2,268 trial participants in that age group were given two smaller doses of the Pfizer vaccine, and the "results provide a strong foundation for seeking authorization of our vaccine for children 5 to 11 years old, and we plan to submit them to the FDA and other regulators with urgency," Pfizer CEO Albert Bourla said in a statement.
The Food and Drug Administration has given emergency approval of the vaccine for kids 12 to 15, and full approval to Americans 16 and older.
"We are eager to extend the protection afforded by the vaccine to this younger population, subject to regulatory authorization, especially as we track the spread of the Delta variant and the substantial threat it poses to children," Bourla said. "Since July, pediatric cases of COVID-19 have risen by about 240 percent in the U.S. — underscoring the public health need for vaccination."
Pfizer is conducting two other ongoing trials, of kids 2 to 5 and children 6 months to 2 years old, and the results for those studies could be in before the end of the year. Nearly 5.3 million children have tested positive for COVID-19 in the U.S., NBC News reports.
You may also like
Did Theranos Lose Afghanistan?
Seven Brides for Seven Brothers star Jane Powell dies at 92
FDA panel overwhelmingly rejects approval of Pfizer vaccine booster shots for people 16 and older

 Yahoo Movies
Yahoo Movies 
