Insulin pens recalled for a problem that might lead to the wrong amount being injected
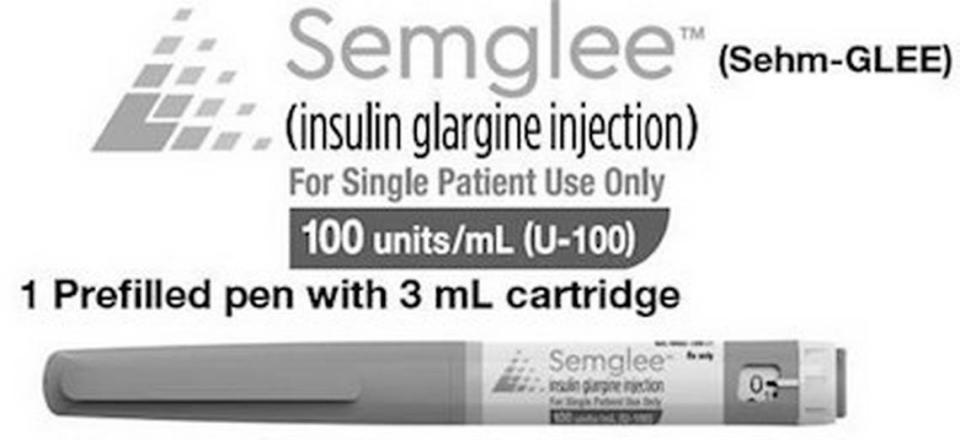
One batch of Semglee prefilled insulin injection pens has been recalled by Mylan Pharmaceuticals for missing labels.
Batch No. BF20003118, expiration August 2022 of Semglee (insulin glargine injection), 100 units/ml (U-100), 3mL prefilled pens come in cartons of five. The cartons are properly labeled. The pens inside might not be.
As stated in the FDA-posted recall notice written by parent company Viatris, in a diabetes patient taking more than one kind of insulin, this “could lead to a mix-up of products/strengths, resulting in administration of the wrong insulin. Administration of the wrong insulin could result in less optimal glycemic control (either high or low blood sugar) which could result in serious complications.”

What you should do if you have the recalled insulin pens?
If you’re a wholesaler, quarantine the batch. Then, Mylan asks that you sent a Microsoft Excel list of retail customers to mylan6069@sedgwick.com. Retailers should quarantine and stop selling the batch.
Consumers should contact Stericycle at 888-843-0255 for a packet to return the pens. If you have questions about the recall, email Viatris at customer.service@viatris.com or call 800-796-9526, Monday through Friday, 8 a.m. to 5 p.m., Eastern time.
If this or any other drug or drug dispenser causes a medical problem, after notifying a medical professional, let the FDA know via its MedWatch Adverse Event page or by filling out a form you can get by calling 800-332-1088. Then, notify the manufacturer.
Company recalls a diabetes drug because it might have too much of a carcinogen
Recall: Microbial contamination in laxative can cause a ‘life-threatening’ infection

 Yahoo Movies
Yahoo Movies 
