A pig's heart has given an ex-con a second chance, but is it ethical?
When David Bennett Sr., 57, agreed to accept a pig heart as a replacement for his own failing one, he took a huge chance.
His surgeon said he couldn't promise whether Bennett would survive an extra day, week or year with the new heart because a gene-edited pig heart had never been tested in a person before.
Some have questioned whether Bennett, who served time in prison for stabbing a man 34 years ago, should have been given a second shot at life.
But it's not yet clear whether Bennett got a gift or a curse, said Art Caplan, a bioethicist at New York University Langone Medical Center.
"I have seen so many first, cutting-edge experiments fail," Caplan said. "What's going on here is more like: How do you pick which test pilot is going to fly the first new dangerous aircraft?"
Past experiences are not encouraging.
The first recipient of a permanent artificial heart, Barney Clark, "suffered horribly, begged his investigator who gave him the artificial heart to shut the thing off and let him die and they wouldn't do it," Caplan said.
Clark died in 1983, 112 days after receiving the device. He never left the hospital.
Jesse Gelsinger, one of the first recipients of gene therapy, endured a "horrible death" when his immune system went into overdrive, Caplan said, and Stephanie Fae Beauclair, a newborn who lived for 21 days in 1984 with a baboon heart beating inside her chest "would have died (anyway), but died more miserably than she would have if she hadn't been in an experiment."
Hank Greely, director of the Center for Law and the BioSciences at Stanford School of Medicine, agreed that being first is a risky position.
"The odds that this is going to help him significantly in the long-run … are very small," Greely said. "Some people will say, 'Yeah, but that's better than dying.' But that's not necessarily true."
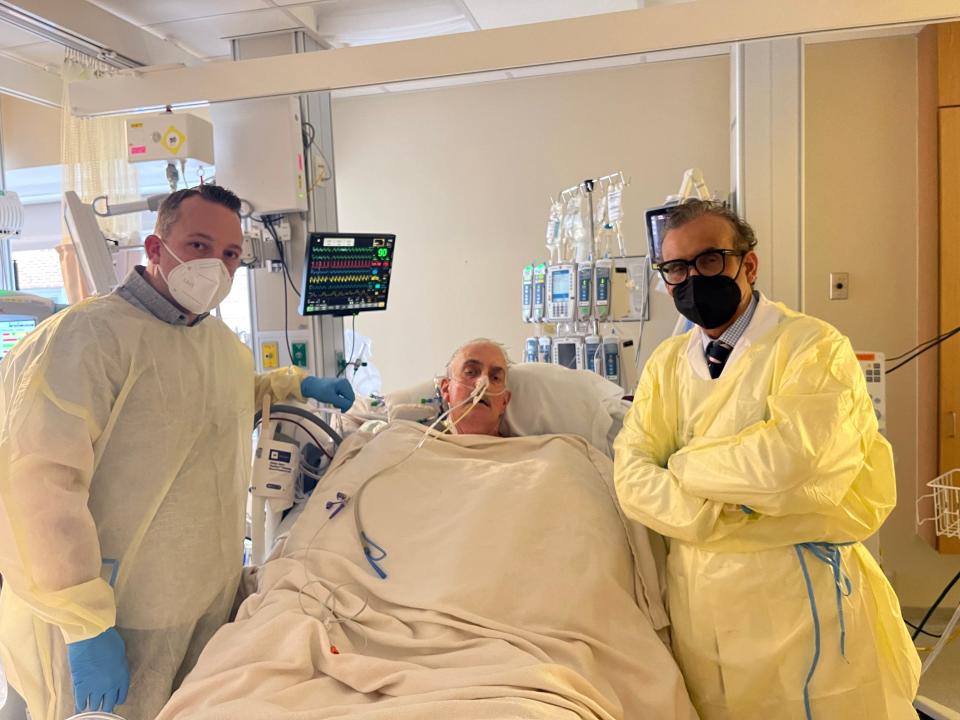
It's not clear exactly what Bennett understood or expected about the surgery, which was carefully explained to him after all his other treatment options had been exhausted. He has not yet spoken publicly after the transplant, though his recovery is going well. But his son said he wanted to live and knew that even if he didn't, the experience would help others.
"Bioethicists love to think that research participants are motivated by altruism," Greely said. "Far more are probably motivated by probably exaggerated hope."
As to whether Bennett's past should have influenced his shot at a new heart, the ethicists and doctors were unanimous: no.
Doctors treat the patient in front of them and don't make judgments about whether the person is deserving or not, said Karen Maschke, a research scholar at The Hastings Center in Garrison, New York, who has a federal grant to study the ethics of using animal organs in people.
"How would we come up with criteria about who we're going to treat or not treat?" Maschke said. Should doctors treat someone convicted of a financial crime but not a violent one? Someone who was wealthy versus someone who wasn't? "How do you make those kind of decisions about people's behaviors?"
'The new heart is still a rock star': Man doing well after receiving first heart from gene-edited pig
The first fully useful dialysis machines in the late 1960s were very expensive and limited in number, Greely said, and hospitals had to decide who would get the life-saving treatment and who wouldn't.
After a hospital in Seattle got publicity for setting up a committee to decide who had the most social worth and therefore deserved to live, Congress authorized Medicare to pay for dialysis for everyone with end-stage kidney disease.
"That's an example of how uncomfortable trying to make life or death decisions based on social worth can be," Greely said.
Caplan said a big question with an experiment like xenotransplantation is "when are you ready to try?"
At NYU, he came up with the idea of first testing the pig organ on a recently deceased person, just as he has suggested that drug companies and gene therapists first try their approach on the recently deceased before the living.
In September, NYU Langone transplant surgeon Robert Montgomery experimentally attached a pig kidney to a recently declared brain-dead person. The kidney seemed to function well and the woman's body didn't mount the kind of immediate immune reaction that has stopped xenotransplantation for decades.
As the researchers in Maryland, Montgomery used a pig whose genes had been edited to prevent rejection, though in his case, only one gene was deleted, while the University of Maryland School of Medicine researchers used a pig with 10 gene edits.
In an essay provided to Newsweek, lead surgeon Bartley Griffith said he'd long been inspired by "firsts" in medicine.
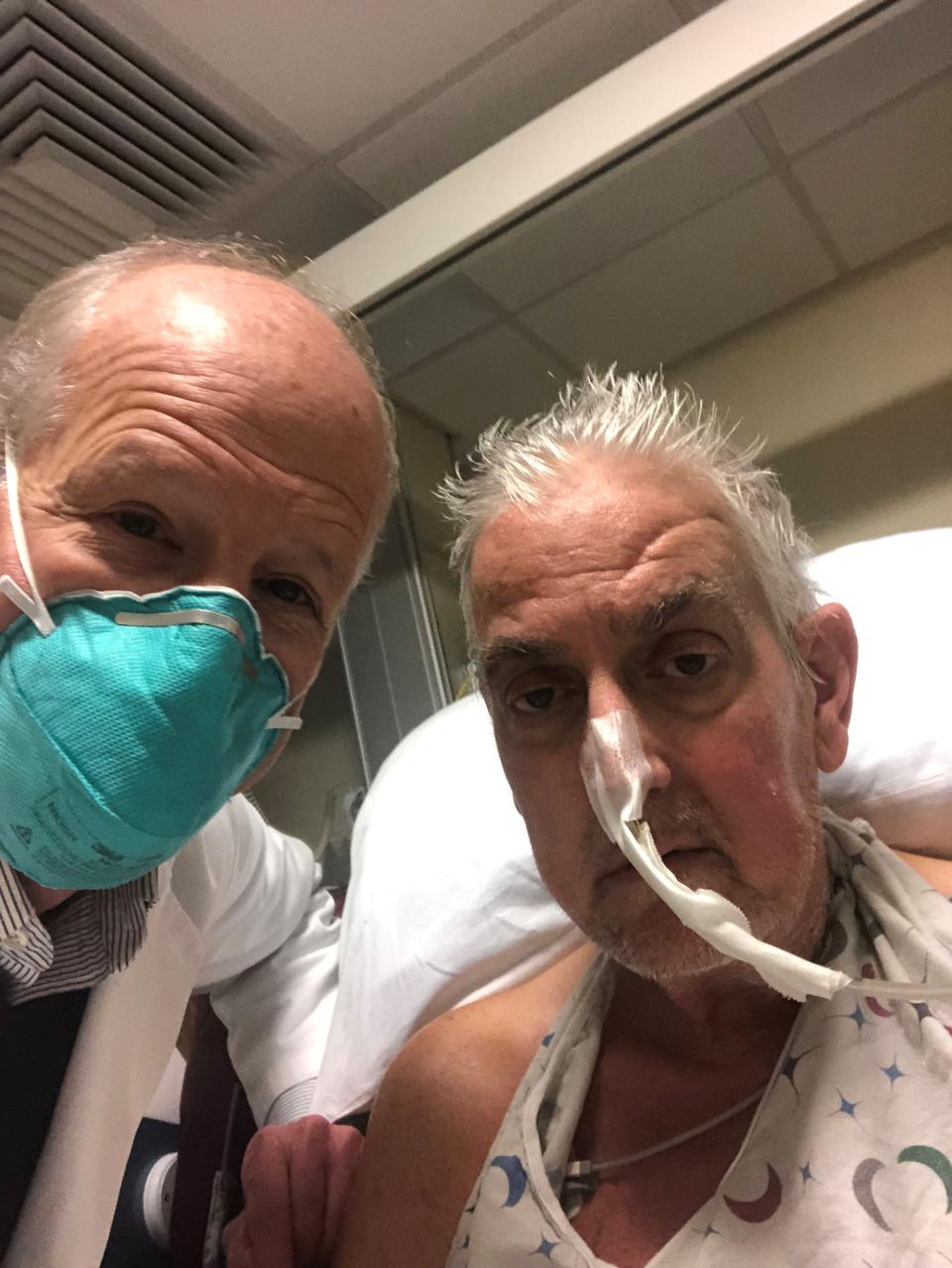
"I came out of that arena of gladiators. People who took on disease and were kicking and fighting their way to new knowledge to help patients," he wrote.
Griffith said all he knew about Bennett was that he'd recently driven a bus for nursing home patients – not about his criminal record.
"We don't look at incarceration history and things like that, I think that's unethical," Griffith wrote. "And it didn't enter into our discussion when we were trying to consider him as a human heart transplant candidate. I felt very confident that none of us knew anything about this particular issue that was so very long ago."
When Bennett woke up after the surgery, he thanked Griffith for saving his life.
"It made me cry. It's simple, but he meant it and it was pretty special," Griffith wrote. "All patients are precious to their surgeons. In the field I work in, you have to have selective memory in order to come to work every day. Not everything works out."
Griffith said he's proud to have been a part of the groundbreaking surgery and sees no ethical issues in placing a human life in front of a pig's.
"It's amazing to be part of something that might make a difference in our whole field. Can you imagine if we had organs on demand for people who needed them? It would be huge," he wrote.
He said he would not agree to use organs from primates, but pigs are "far away from humans" genetically. "I'm honestly respectful of the opposite opinion. We have to always be reminded that this is a living creature and we're taking its life."
The ethicists said they see no problem sacrificing pigs for the sake of extending human lives.
BACKGROUND: First-ever pig-to-human heart transplant offers hope for thousands in need of organs
Maschke said she hopes the scientific world will eventually move away from using animals, but that time hasn't yet arrived.
Greely said he loves bacon and "if eating an animal is OK when there are lots of substitutes for that protein, using an organ to keep someone alive when there aren't substitutes" should be OK too.
Caplan agreed.
"The place to worry about animal welfare is breakfast not the medical use of pigs," Caplan said.
"Those pigs, the ones that are genetically engineered are raised on special farms, obviously have to be very healthy, can't be stressed and never suffer – they kill them, that's true, but it's not the same at all as factory farming and some of the terrible practices that millions and millions and millions of pigs are subjected to," Caplan said. "The equation for me comes out (for) saving human lives."
Caplan also quickly disposed of the argument some people have made that extending human lives will contribute to overpopulation.
"If you start down that road, you have to close down medicine," he said. "The route to controlling overpopulation is doing something about reproduction, not letting the sick die."
Caplan said he also hopes that Bennett understood that he would be subjected to public scrutiny by going public with his identity. The hospital might have done better by keeping his identity a secret, Caplan said. "Maybe if you protect patient identity, in terms of privacy, you don't create this kind of whole tragic scenario," where people are questioning whether Bennett deserved the heart.
Shouldn't the public know about the background of a high-profile patient?
"The answer is no," Caplan said.
Such publicity may deter future patients, and at minimum, a patient needs to be told about the potential consequences before they agree to make their identity public. "It's something to think about from the point of view of protecting the patient," Caplan said.
Maschke said she has ethical questions about the procedure being allowed under the Food and Drug Administration's "compassionate use" criteria, rather than as part of a clinical trial.

Although she trusts the scientific integrity of the University of Maryland team, she said there is no requirement now that they reveal details of their protocol or their criteria for selecting a patient, which would be a contribution to the scientific field. More can be learned as part of a clinical trial than a one-off procedure, she said.
It's not clear what the FDA will do the next time doctors request permission to transplant a pig organ in an effort to save a person's life. "That pathway is not without concern," she said.
Several groups are poised to request approval for clinical trials in xenotransplantation as soon as this year, she said. But even if that approval comes soon, it will be a long time before transplanting a pig organ into a person will be shown to be safe and effective.
"Research physicians always want to find the next cure. That's admirable," she said, noting that much of their research is federally funded. "But there's always that fine line between 'are you moving too fast into the patient population' – (or) are you doing controlled, rigorous research, called clinical trials, which take longer than you'd like."
There are many open and as yet unanswerable questions about pig-to-human transplants, Maschke said.
Does success mean as good as a human-to-human transplant or something else? Will pig hearts work as well as pig kidneys or pig livers? What is the patient's quality of life with a pig organ and the immune suppression protocol followed in Bennett's case? What criteria will be used to determine if someone should get a pig or a human organ?
"The answer to those questions all have implications for use of resources to support this kind of transplantation," she said.
Contact Karen Weintraub at kweintraub@usatoday.com
Health and patient safety coverage at USA TODAY is made possible in part by a grant from the Masimo Foundation for Ethics, Innovation and Competition in Healthcare. The Masimo Foundation does not provide editorial input.
This article originally appeared on USA TODAY: Pig heart transplant gives ex-con a second chance. But is it ethical?

 Yahoo Movies
Yahoo Movies 