Drugs recalled for overdose threat — stronger pain pills put in weaker pills’ bottles
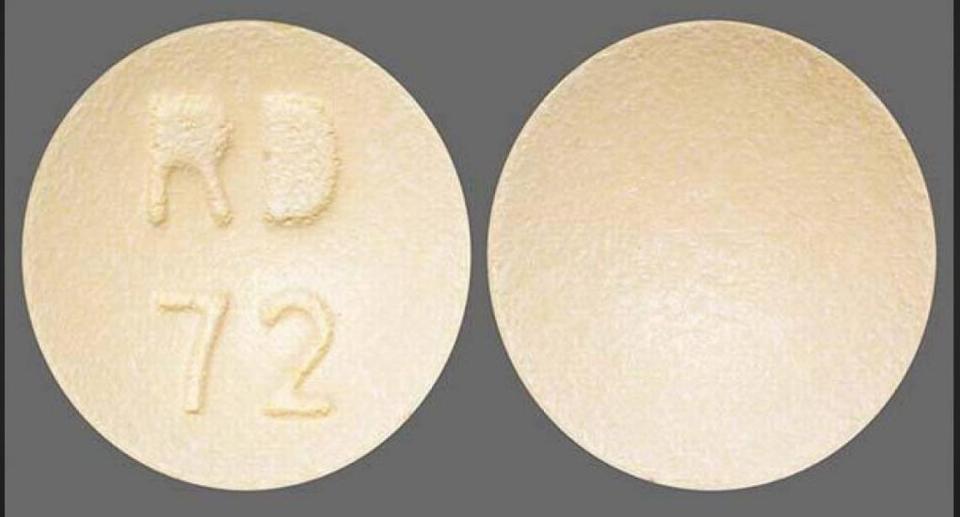
A packing mistake that can have deadly consequences caused Bryant Ranch Prepack to recall one lot each of Morphine Sulfate 30 mg Extended-Release tablets and Morphine Sulfate 60 mg Extended-Release tablets.
The 60 mg pills were put in bottles labeled for the 30 mg dose, and vice-versa.
“Patients prescribed the 30 mg dose who receive the 60 mg dose could be at risk for overdose and death,” the risk statement of the Bryant Ranch-written recall notice says. “Patients prescribed the 60 mg dose who receive the 30 mg dose may experience withdrawal and untreated pain if the dose given is too low.”
This covers lot No. 179642, expiration date 11/30/2023, NDC No. 63629-1088-01 for the 30 mg tablets and lot No. 179643, expiration date 8/31/2023, NDC No. 63629-1089-01 for the 60 mg tablets.

If you have either of these tablets, either as a patient or as a seller, stop using them or selling them and reach out to Bryant Ranch by email at cs@brppharma.com or by calling 877-885-0882, Monday through Friday, 10:30 a.m. to 8 p.m., Eastern time. Use that contact information if you have questions about this recall.
If this or any drug causes a problem, after notifying a medical professional, let the FDA know via its MedWatch Adverse Event page or by filling out a form you can get by calling 800-332-1088. Then, notify the manufacturer.

 Yahoo Movies
Yahoo Movies 
